Uncovering Physical Contributors to Hypertension
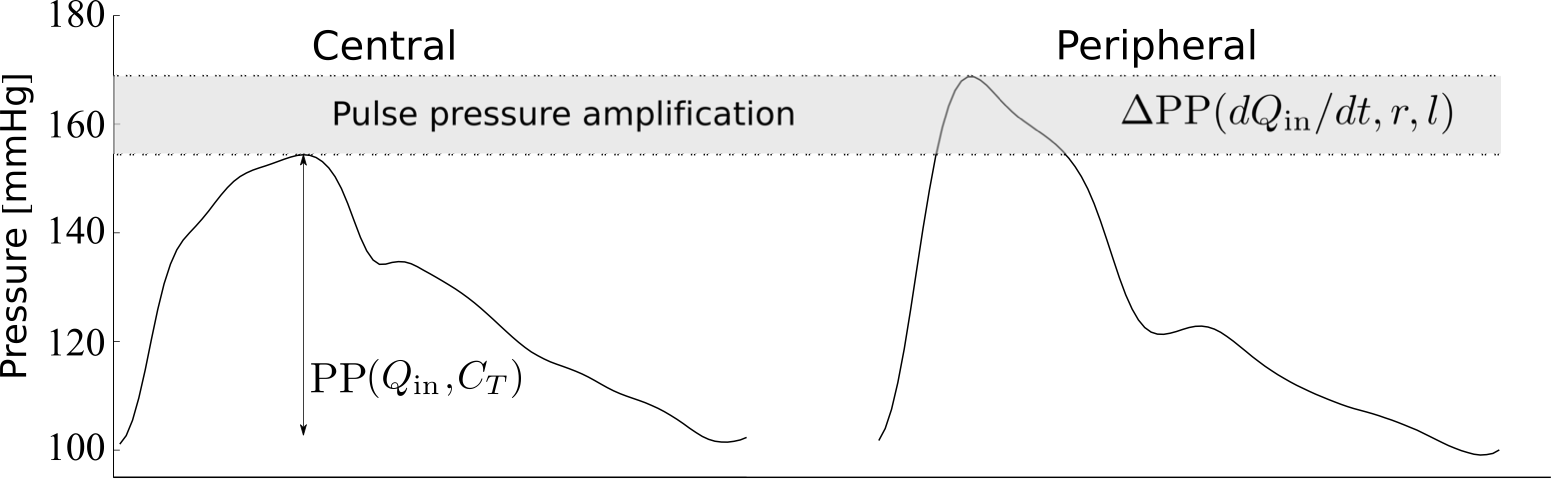
High arterial blood pressure (BP) is one of the most important causes of global morbidity and mortality. Increased central pulse pressure (cPP; i.e. the amplitude of the aortic BP wave) is the major cause of incident hypertension in middle-aged to older persons. We are using haemodynamics to (i) identify and quantify individual cardiovascular contributions to cPP and its amplification to the periphery (where it is normally measured), and (ii) uncover the underlying physical mechanisms leading to increased central and peripheral pulse pressure.
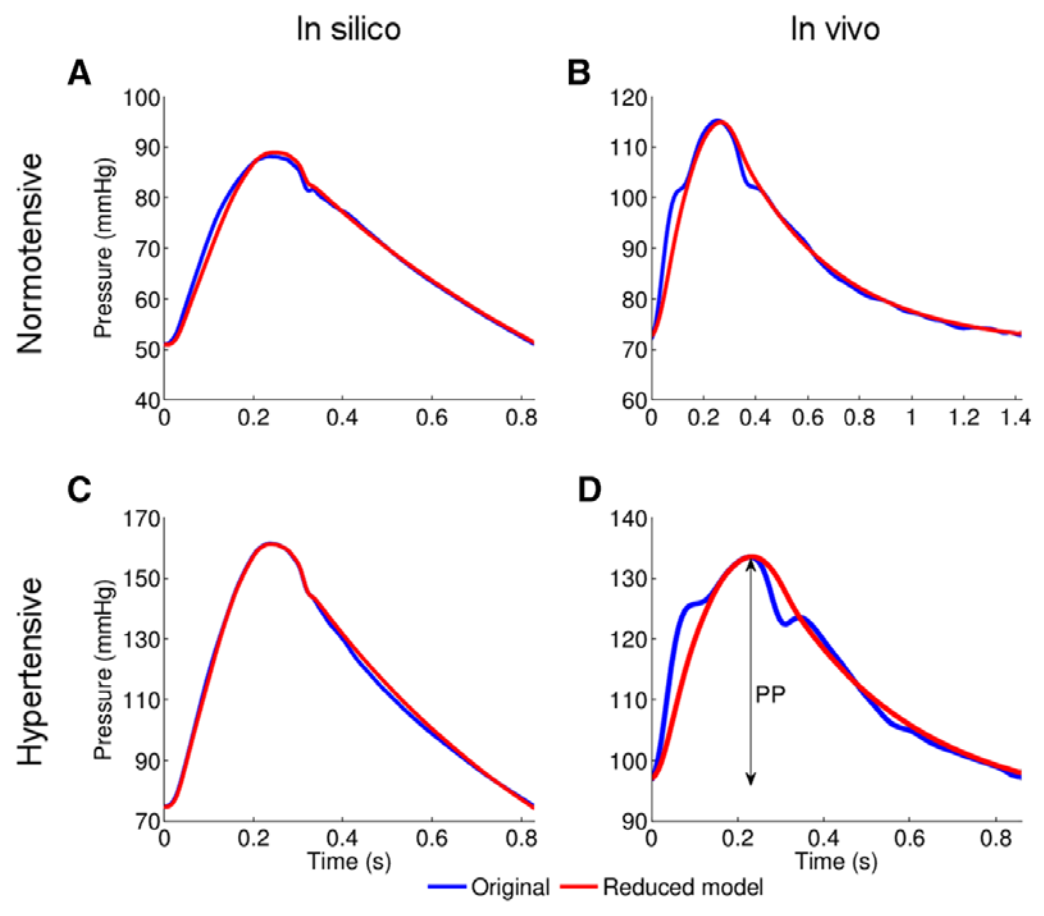
 We have shown that ventricular dynamics and aortic stiffness play a key role in raising pulse pressure with hypertension. Since drugs with a specific action to reduce arterial stiffness are not yet available, interventions to modulate ventricular dynamics might be useful in preventing/treating systolic hypertension. Ventricular dynamics is also a main determinant of pulse pressure amplification which offers opportunity for noninvasive assessment of ventricular health by analysing the peripheral pulse wave signal. More specifically:
We have shown that ventricular dynamics and aortic stiffness play a key role in raising pulse pressure with hypertension. Since drugs with a specific action to reduce arterial stiffness are not yet available, interventions to modulate ventricular dynamics might be useful in preventing/treating systolic hypertension. Ventricular dynamics is also a main determinant of pulse pressure amplification which offers opportunity for noninvasive assessment of ventricular health by analysing the peripheral pulse wave signal. More specifically:
 We have shown that ventricular dynamics and aortic stiffness play a key role in raising pulse pressure with hypertension. Since drugs with a specific action to reduce arterial stiffness are not yet available, interventions to modulate ventricular dynamics might be useful in preventing/treating systolic hypertension. Ventricular dynamics is also a main determinant of pulse pressure amplification which offers opportunity for noninvasive assessment of ventricular health by analysing the peripheral pulse wave signal. More specifically:
We have shown that ventricular dynamics and aortic stiffness play a key role in raising pulse pressure with hypertension. Since drugs with a specific action to reduce arterial stiffness are not yet available, interventions to modulate ventricular dynamics might be useful in preventing/treating systolic hypertension. Ventricular dynamics is also a main determinant of pulse pressure amplification which offers opportunity for noninvasive assessment of ventricular health by analysing the peripheral pulse wave signal. More specifically:
- cPP is mainly determined by total arterial compliance (inversely associated with central arterial stiffness) and ventricular dynamics: ventricular ejection (corresponding to aortic flow) and rate of ejection (Hypertension, 2017);
- Ventricular dynamics account for a relatively large proportion of the increased cPP, suggesting that cardiac dynamics are as relevant as vascular properties. Pressure reflection from the vasculature may be less important in raising cPP than previously thought and, hence, cardiac properties should be involved in the treatment strategy (Hypertension, 2017);
- Increased cPP during inotropic stimulation and in essential hypertension results primarily from the pressure forward wave (Hypertension, 2014);
- The amount of reflection of the forward pressure wave from the peripheral vasculature contributes little to increased cPP in hypertension (Hypertension, 2017), underlining the importance of ventricular dynamics and proximal aortic pulse wave velocity as the major determinants of variation in cPP;
- Pressure wave generation and reflection at the aortic root can be quantified by a time-varying emission coefficient that is directly proportional to aortic flow and whose peak increases with increasing cPP. Ventricular-aortic coupling is the main determinant in cPP elevation (IEEE Trans Biomed Eng, 2021);
- The age-related increase in cPP is driven mainly by an increase in aortic stiffness and altered pattern of ventricular ejection. Consequently, conditions and drugs that influence cardiac function may influence pulse wave morphology independent of arterial function (Hypertension, 2019);
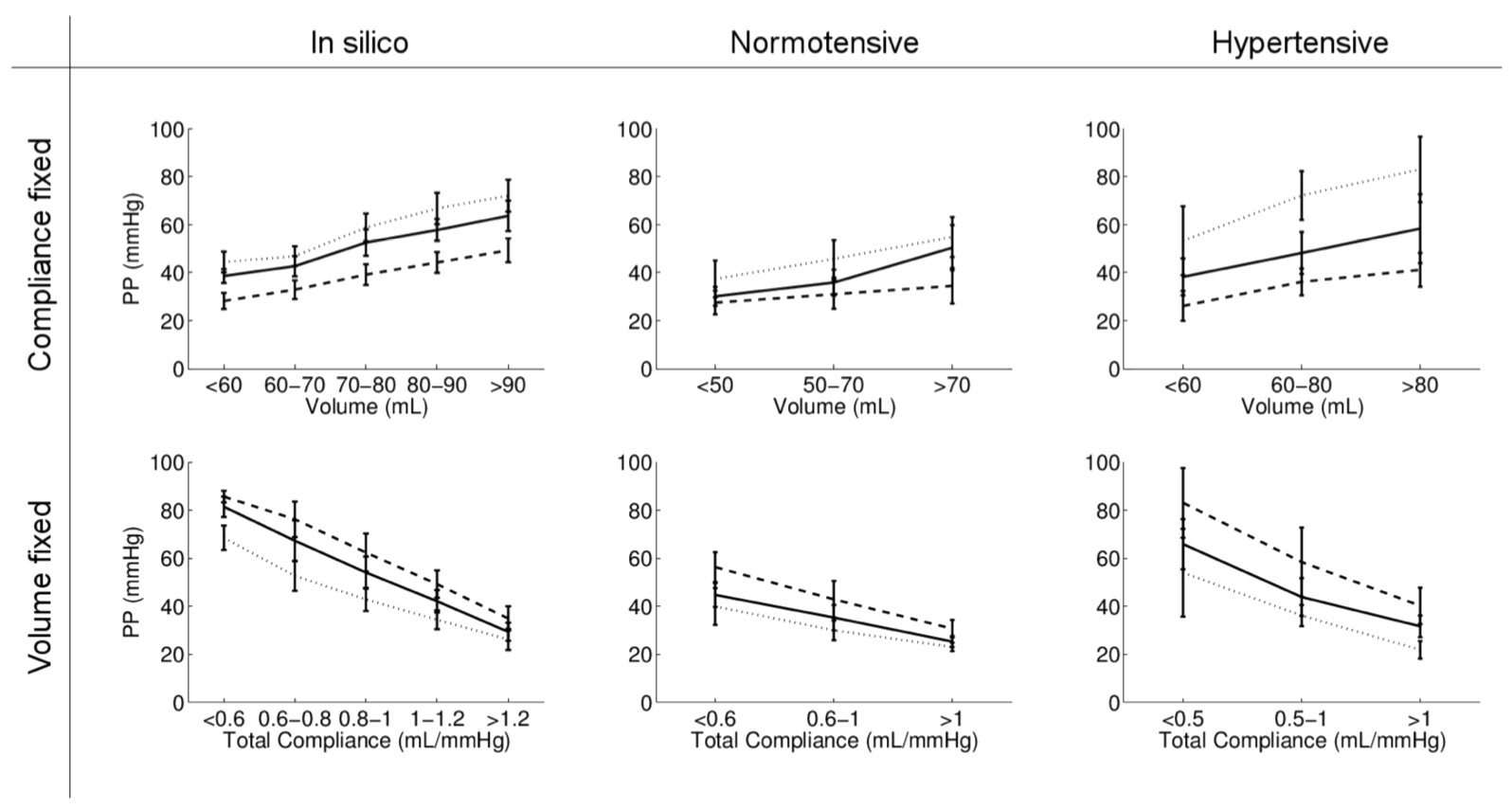
- Ventricular dynamics play a crucial role in pulse pressure amplification to the periphery, both theoretically (Frontiers Physiol, 2021) and experimentally using the model shown below (Am J Physiol, 2017). The rate of change of aortic flow with time in late systole (strongly correlated with ventricular ejection dynamics), and vessel radius and length from the aortic root to the periphery are the main cardiovascular properties that determine pulse pressure amplification (Frontiers Physiol, 2021);
- Peripheral systolic BP (pSBP, used to assess clinical risk associated with hypertension and guide acute clinical care) is determined mainly by the first shoulder of the central BP waveform (P1) and the rate of rise of the central waveform (dP/dt), which are different from the determinants of central systolic BP (P2). This may be a potential mechanism why BP-lowering drugs like β-blockers may have differential effects on central and peripheral BP (Am J Physiol, 2021).
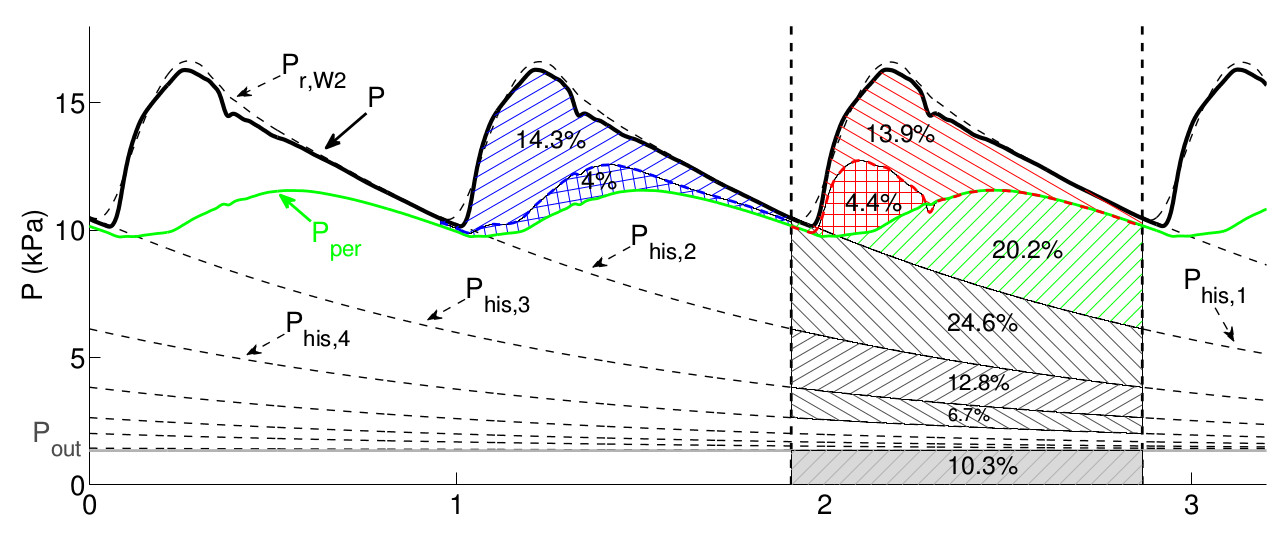
- Separate the aortic blood pressure wave into well-defined components with different biophysical and temporal origins, allowing quantification of the relative contributions from cardiac and vascular properties (IEEE Trans Biomed Eng, 2021; Ann Biomed Eng, 2016);
- Quantify the pressure wave generation and reflection at the aortic root using a novel time- varying emission coefficient (IEEE Trans Biomed Eng, 2021);
- Reduce the number of 1-D model arterial segments by lumping them into 0-D models, which shows that the cPP of a distributed multisegment model of the arterial tree can be described by a Windkessel model, with little loss of fidelity and full theoretical understanding of the physical meaning of the model parameters (J R Soc Interface, 2018; Am J Physiol, 2015);
- Create populations of thousands of virtual subjects for in silico evaluation of pulse wave indices and algorithms (Symmetry, 2021; Am J Physiol, 2015 and 2019; J Biomech, 2016);
- Obtain analytical solutions for the blood pressure and flow waveforms (Frontiers Physiol, 2021; Ann Biomed Eng, 2016).