Arterial Stiffness Assessment from Pulse Waves
As people age, arteries stiffen, raising systolic and pulse pressures, increasing mechanical stress on vital organs such as the heart, brain, and kidneys, potentially contributing to disease. Large artery stiffness independently predicts cardiovascular morbidity and mortality. Direct measurement is challenging, but indirect assessments are possible; e.g. by measuring the velocity of propagation of the arterial pulse wave or by analysing pulse wave morphology. Moreover, quantification of aortic stiffness facilitates central blood pressure estimation.
We have studied various methods for assessing aortic stiffness and shown the following results with respect to two-point methods for estimating pulse wave velocity (PWV):
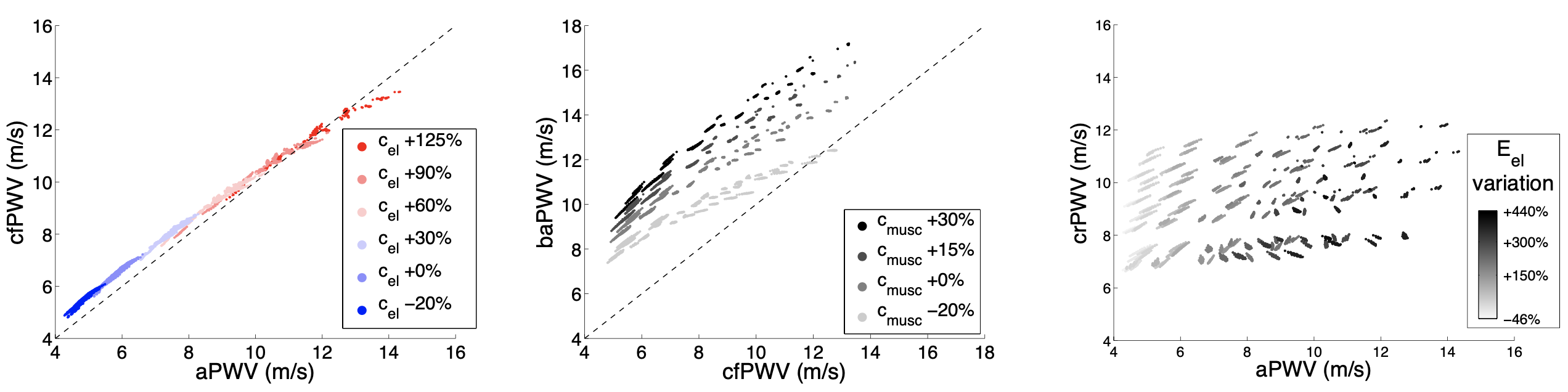
- Carotid-femoral PWV is an accurate indicator of aortic stiffness, exhibiting a strong correlation with aortic PWV (Am J Phys, 2015; J Biomech, 2016) and aortic Young's modulus (Am J Phys, 2023);
- Brachial-ankle PWV over-estimates aortic PWV since it is related to the stiffness and geometry of both elastic and muscular arteries (Am J Phys, 2015; J Biomech, 2016);
- Both carotid-radial and femoral-ankle PWV do not capture aortic stiffening (Am J Phys, 2015; J Biomech, 2016);
- Methods using ascending and descending aorta flows outperformed those using carotid and femoral BP waves (Am J Phys, 2021);
- The accuracy of foot-to-foot methods is compromised by late diastolic reflected waves (Am J Phys, 2015; J Biomech, 2016);
- Noise, temporal resolution, and waveform shape similarity should be considered when selecting an algorithm for estimating transit time (Ann Biomed Eng, 2013);
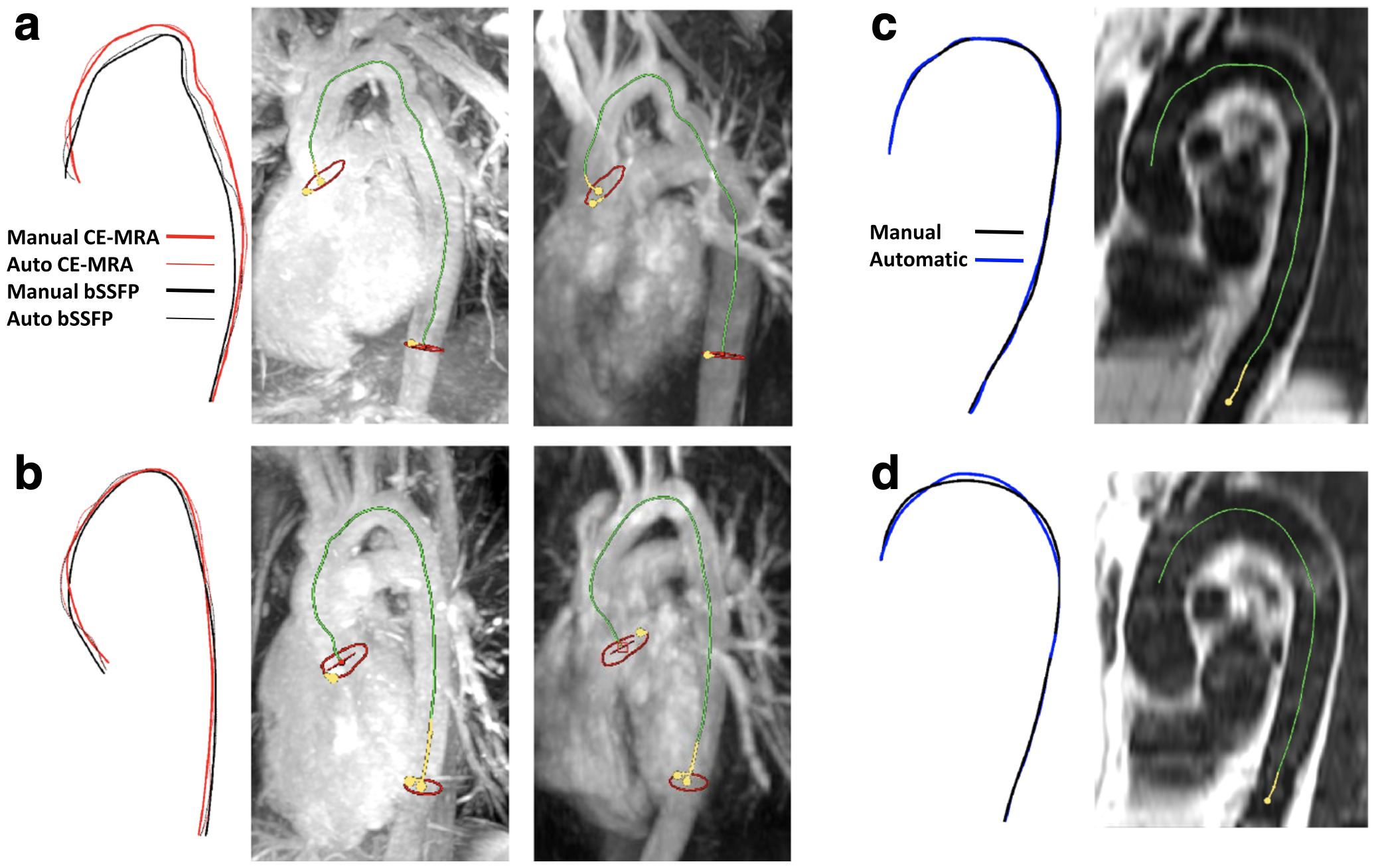
- Automatic tracking of the aorta's 3-D centreline in three commonly used cardiovascular MR sequences yields more accurate distance measurements than those from a 2-D oblique-sagittal plane (J Cardiovasc Magn Reson, 2017).
- There are strong correlations with aortic PWV of indexes calculated from pulse wave morphology. However, these indexes are influenced by other cardiovascular properties (such as heart rate and stroke volume) which may affect their ability to stratify patients (Am J Physiol, 2023 & 2019);
- PPG-based indices show promise for daily VA monitoring (Am J Physiol, 2023);
- Machine learning algorithms enable accurate PWV estimation from a single peripheral pulse wave (PLoS One, 2021);
- Loop methods' accuracy diminishes in arterial sites with significant visco-elastic effects and reflected waves (J Biomech, 2016 & 2011);
- The sum-of-squares method is strongly influenced by reflected waves and is more accurate at proximal locations (J Biomech, 2016 & 2011);
- PWV estimates obtained using the PU-loop method in the ascending aorta can be corrected for early-systolic pulse wave reflections (Int J Numer Meth Biomed Eng, 2014);
- PWV can be accurately estimated using a method insensitive to early-systolic reflected waves and blood flow velocity errors. This approach also calculates an accurate cross-sectional average velocity waveform from Doppler ultrasound data, regardless of scaling errors (Physiol Meas, 2017).
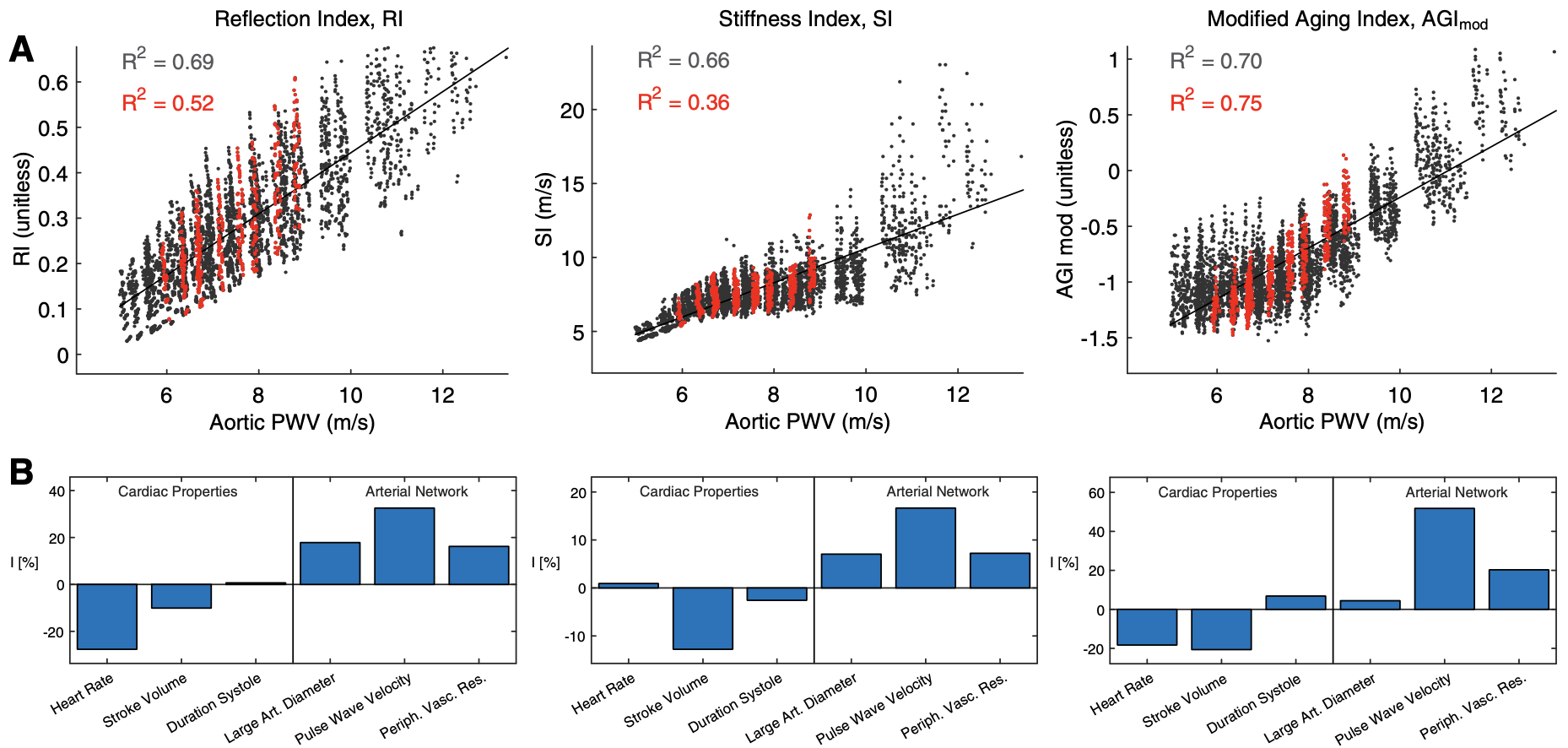
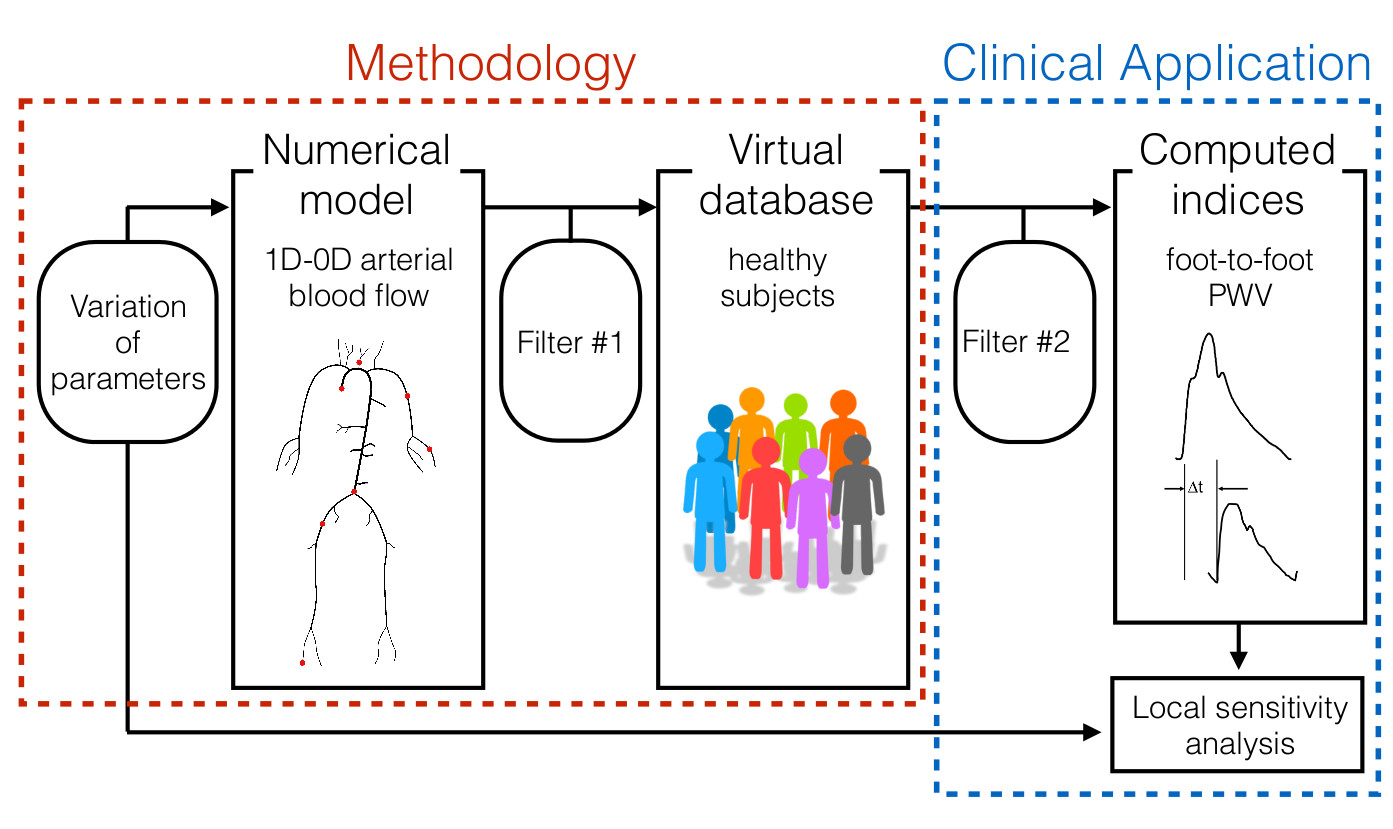
- Create populations of thousands of virtual subjects for in silico evaluation of algorithms and pulse wave indices of arterial stiffness assessment (Am J Physiol, 2015 and 2019; J Biomech, 2016);
- Extract over 30 features proposed in the literature for assessing the stiffness of both large and small arteries from a single pulse wave signal (Am J Physiol, 2019; Physiol Meas, 2018). This algorithm, called PulseAnalyse, is available for download;
- Calculate the transit time between two pulse wave signals (blood pressure or flow waves) using foot-to-foot, least squared difference, or cross-correlation algorithms (Ann Biomed Eng, 2013). The algorithm is also available for download.